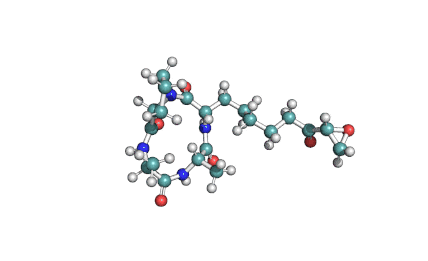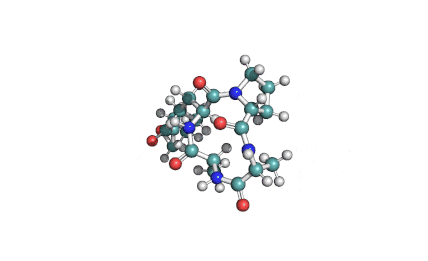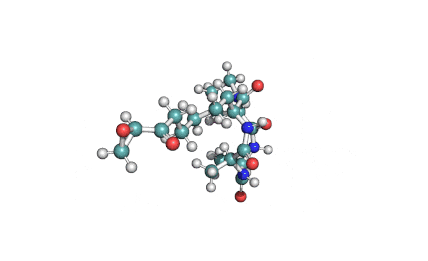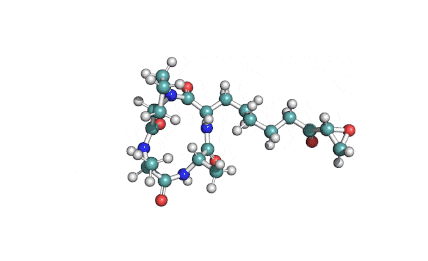
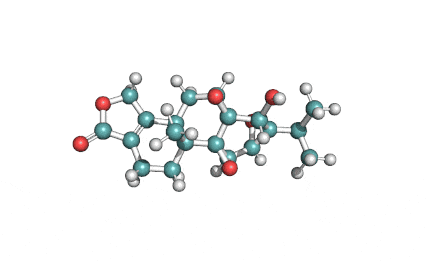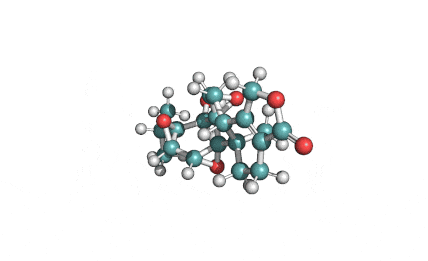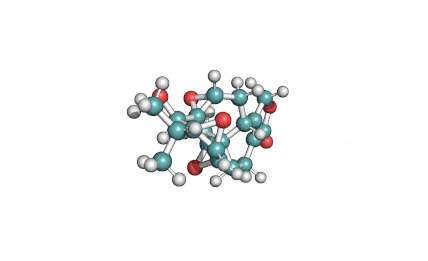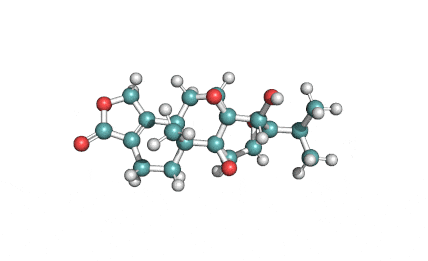
HC toxin
Details
Specifications
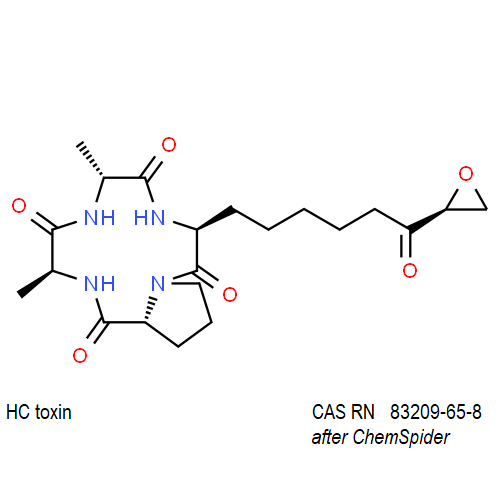
Chemical identification
Synonyms:
- HC-Toxin
Helminthosporium Carbonum Toxin I
Chemical names:
IUPAC: (3S,6R,9S,14aR)-3,6-Dimethyl-9-{6-[(2S)-2-oxiranyl]-6-oxohexyl}decahydropyrrolo[1,2-a][1,4,7,10]tetraazacyclododecine-1,4,7,10-tetrone
RTECS#
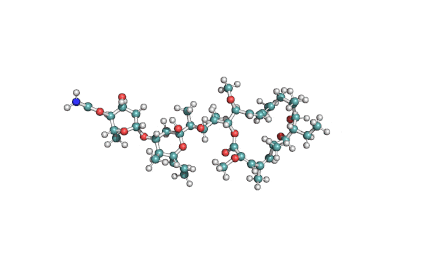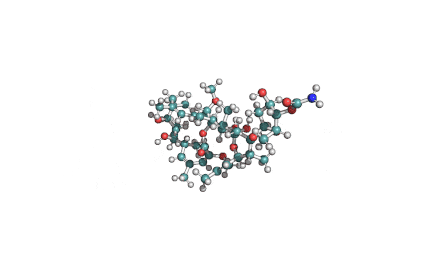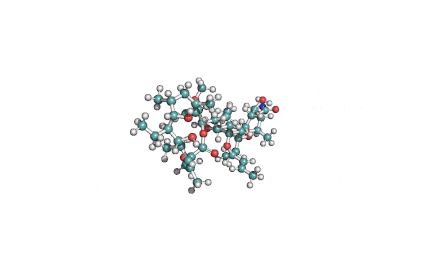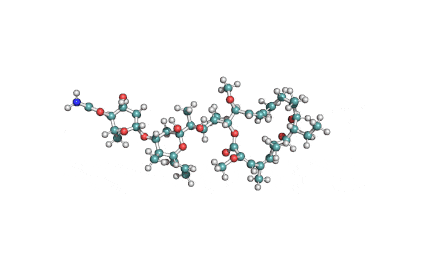
Cyclic tetrapeptide of structure cyclo(D-Pro-L-Ala-D-Ala-L-Aeo), where Aeo stands for 2-amino-9,10-epoxi-8-oxodecanoic acid. HC toxin is a potent, cell-permeable inhibitor of histone deacetylase (HDAC).
Further Information
Cyclic oligopeptide
Composition
Special Info
Other Fields