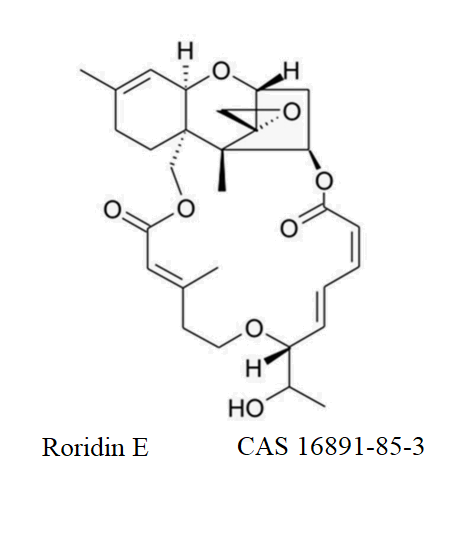
Roridin E
Details
Specifications
Chemical identification
Synonyms:
- Roridin E
- Verrucarin A, 2',3'-didehydro-7'-deoxo-2'-deoxy-7'-(1-hydroxyethyl)-, (2'E,7'R(R))-
Chemical names:
IUPAC: Verrucarin A, 2',3'-didehydro-7'-deoxo-2'-deoxy-7'-(1-hydroxyethyl)-, (2'E,7'R(R))-(1R,3R,8R,12E,17R,18E,20Z,24R,25S,26S)-17-[(1R)-1-hydroxyethyl]-5,13,25-trimethylspiro[2,10,16,23-tetraoxatetracyclo[22.2.1.03,8.08,25]heptacosa-4,12,18,20-tetraene-26,2'-oxirane]-11,22-dione
T3DB: T3D3711
RTECS# YX9821500
Roridin E is trichothecene toxin produced by molds from Fusarium, Myrothecium, and Stachybotrys genera.
Further Information
Composition
Supply related information
Special Info
Other Fields