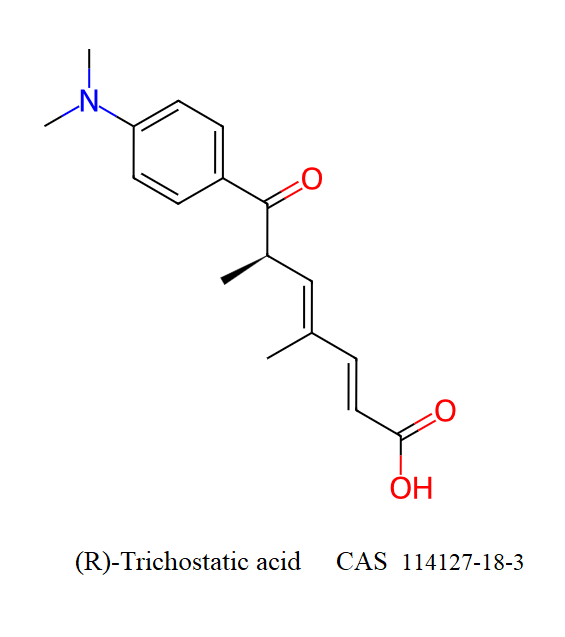
(R)-Trichostatic acid
Details
Specifications
Clear colorless to yellow solution at 20mg/ml of DMSO
Chemical identification
Synonyms:
- (R)-trichostatic acid
- (+)-trichostatic acid
- Trichostatic acid
Chemical names:
IUPAC:
- (2E,4E,6R)-7-[4-(dimethylamino)phenyl]-4,6-dimethyl-7-oxohepta-2,4-dienoic acid
- (R)-trichostatic acid
ECHA Number: 834-835-4
Further Information
Composition
Supply related information
Special Info
Other Fields