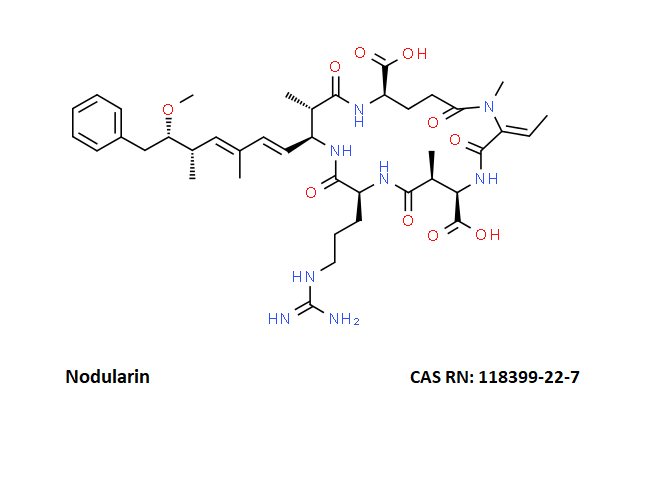
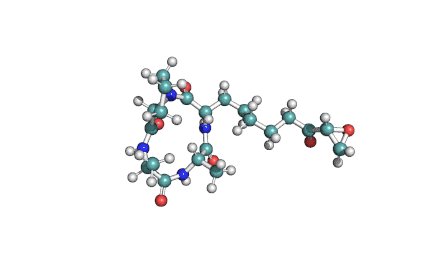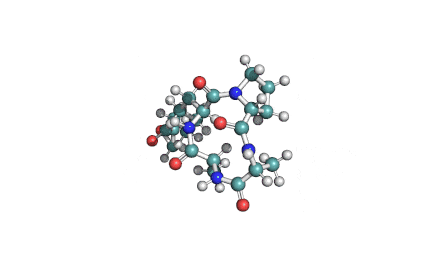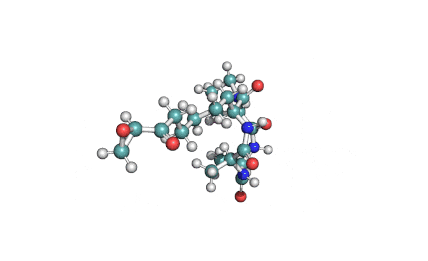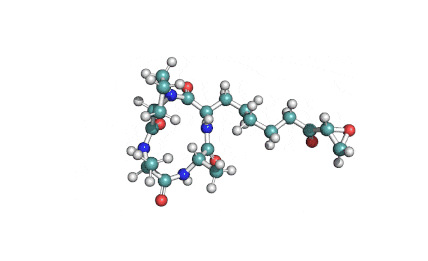
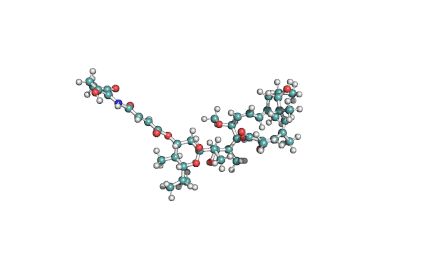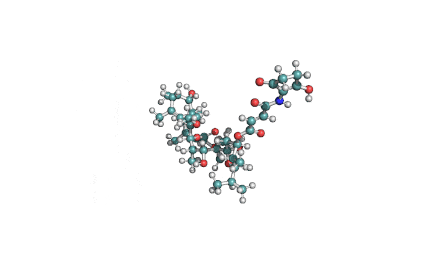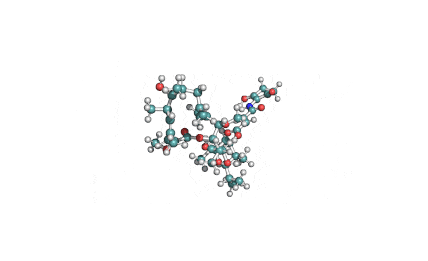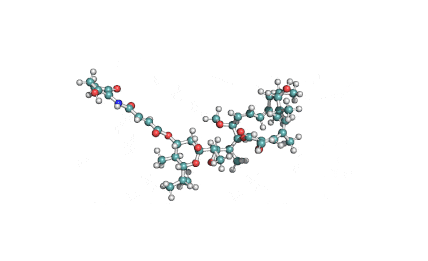
Nodularin
Details
Specifications
Clear colorless to slight brown at 2mg/ml of Methanol
Chemical identification
- Nodularin
- Nodularin R
- Cyclo[(Z)-2,3-didehydro-N-methyl-2-aminobutanoyl-erythro-3-methyl-D-β-aspartyl-L-arginyl-(2S,3S,4E,6E,8S,9S)-4,5,6,7-tetradehydro-9-methoxy-2,6,8-trimethyl-10-phenyl-3-aminodecanoyl-D-γ-glutamyl]
IUPAC:
(2Z,5R,6S,9S,12S,13S,16R)-9-[3-(diaminomethylideneamino)propyl]-2-ethylidene-12-[(1E,3E,5S,6S)-6-methoxy-3,5-dimethyl-7-phenylhepta-1,3-dienyl]-1,6,13-trimethyl-3,7,10,14,19-pentaoxo-1,4,8,11,15-pentazacyclononadecane-5,16-dicarboxylic acid
HSDB: 7749
RTECS# GU2294250
Nodularins are potent toxins produced by the cyanobacterium Nodularia spumigena . This aquatic, photosynthetic cyanobacterium forms visible colonies that present as algal blooms in brackish water bodies.
Further Information
- Methanol 2mg/ml
- MeOH:Water 1:1 v:v 1mg/ml
- EtOH, DMSO 10mg/ml
- Cyanotoxin
- Cyclic Oligopeptide
- Non ribosomal peptide
- Non-protein-building aminoacids
- Potent inhibitor of protein phosphatases 1 and 2A (PP1 and PP2A).
- Useful for affinity purification of PP2A Similar to Microcystin-LR (ICN Cat. No. 158379), but with increased water solubility.
- Ref: 1. J. Cancer Res. Clin. Oncol., 116, 609-614 (1990).
Composition
Special Info
Other Fields