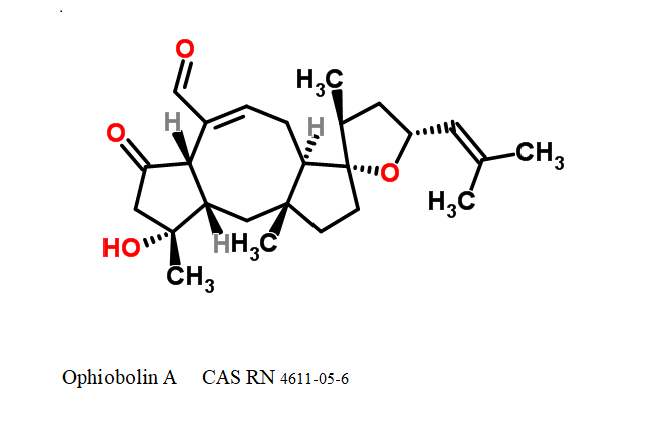
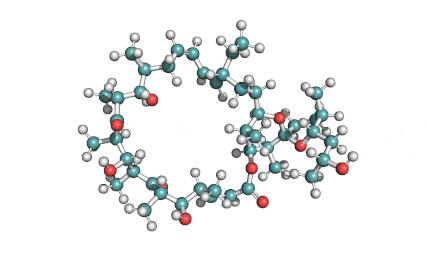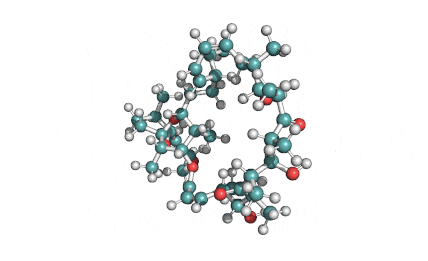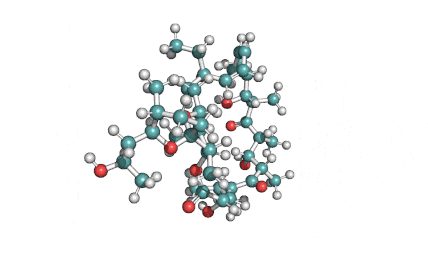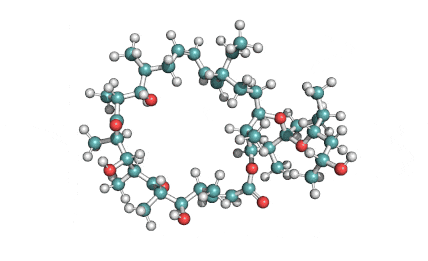
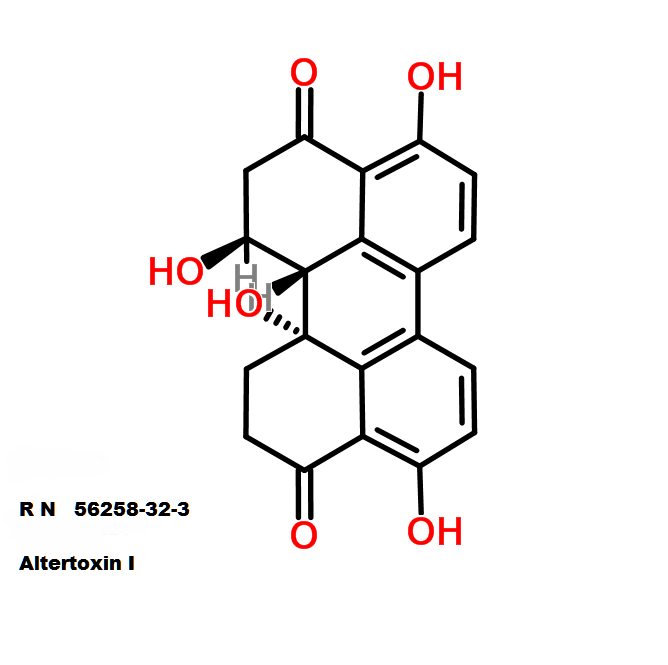
Ophiobolin A
Details
Specifications
Chemical identification
Synonyms:
- Ophiobola-7,19-dien-25-al, 14,18-epoxy-3-hydroxy-5-oxo-, (18R)- (8CI)
- Ophiobolin A
- Cochliobolin
- Ophiobolin;
- Cochliobolin A
IUPAC:
- (7E,18R)-3-Hydroxy-5-oxo-14,18-epoxyophiobola-7,19-dien-25-al
RTECS# : RL1576000.
Sesterterpene, natural inhibitor of Calmodulin, exhibiting anti-cancer, antibacterial properties. Phytotoxin from a plant pathogen.
Further Information
Soluble in ethanol, methanol, DMF or DMSO. Poor water solubility
Composition
Supply related information
Special Info
Other Fields