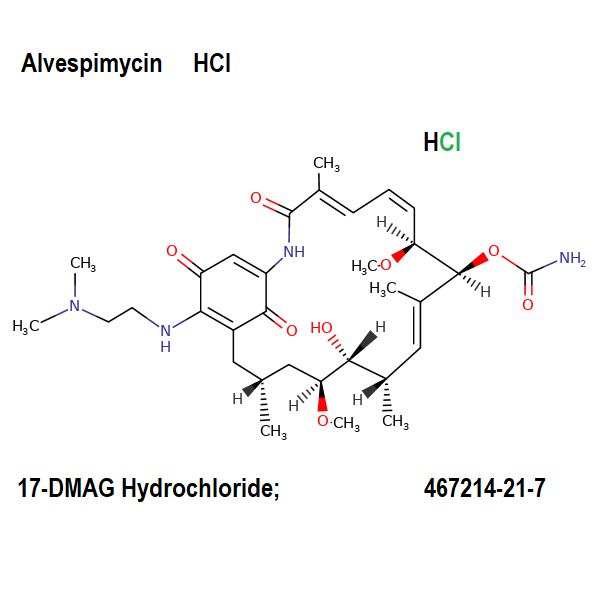
17-DMAG HCl
Details
Specifications
Chemical identification
Synonyms:
- 17-DMAG HCl
- Alvespimycin HCl
- 17-Dimethylaminoethylamino-17-demethoxygeldanamycin Hydrochloride
IUPAC Name:
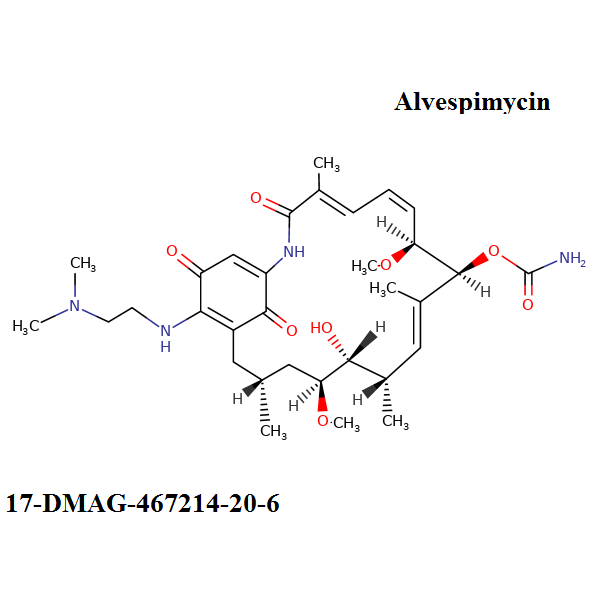
[(4E,6E,9S,10E,12S,13R,16R)-19-[2-(dimethylamino)ethylamino]-13-hydroxy-8,14-dimethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl] carbamate;hydrochloride
RTECS: LX8922000
17-DMAG, also known as Alvespimycin, is a semisynthetic derivate of Geldanamycin, of less hepatotoxicity, improved water solubility.
17-DMAG is an ansamycin antibiotic which binds to Hsp90 (Heat Shock Protein 90) and alters it function.
Further Information
DMSO, Methanol, Ethanol
- Benzoquinone ansamycin class antibiotic
- Geldanamycin derivate
- HSP90 inhibitor
HSP-90 inhibitor; potential anti-cancer drug
Composition
Supply related information
Special Info
Other Fields