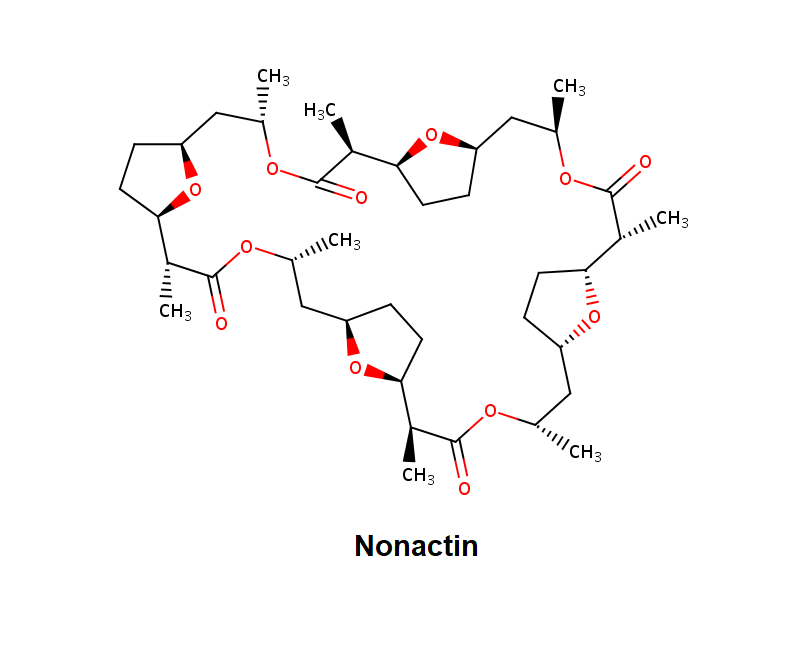
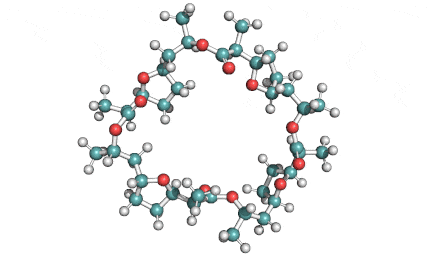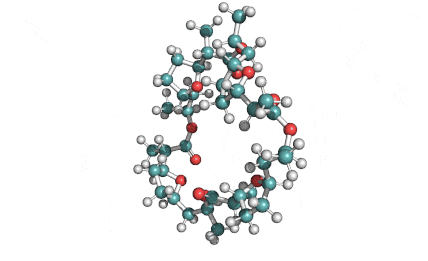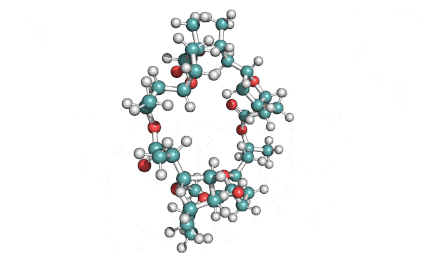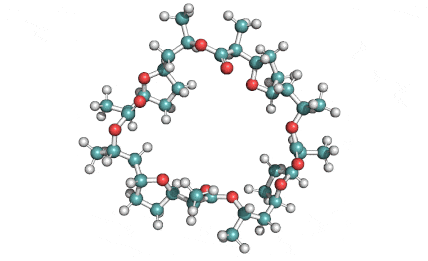
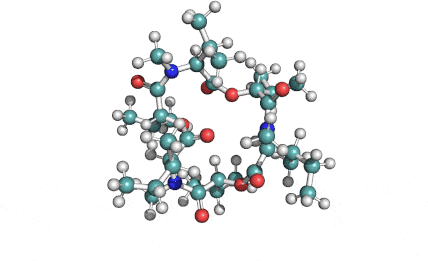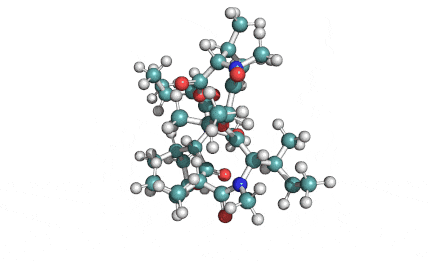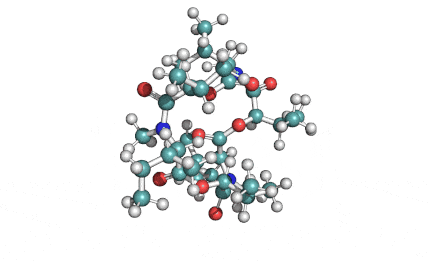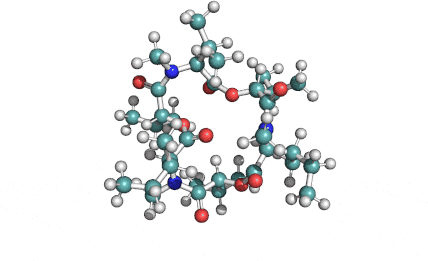
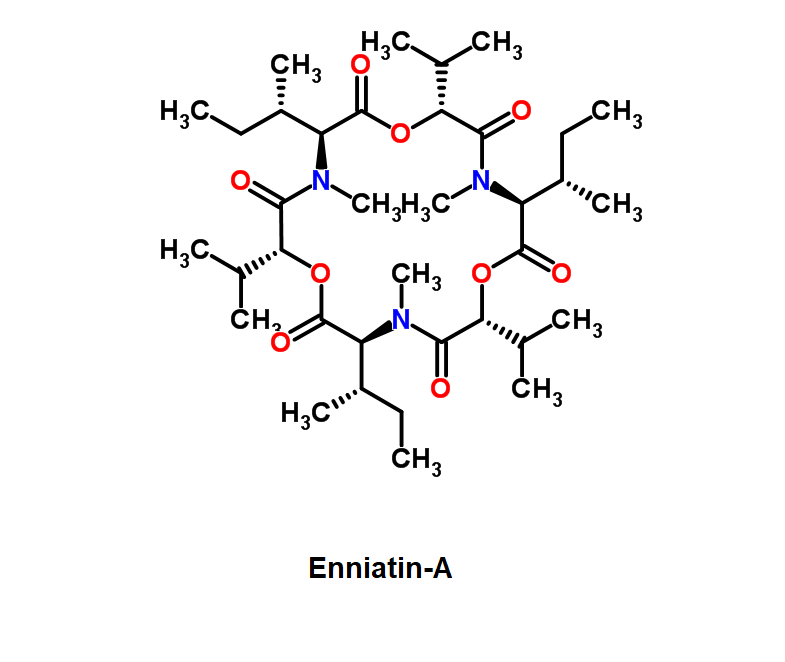
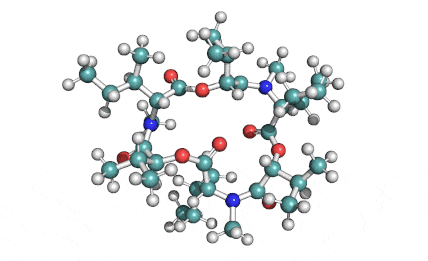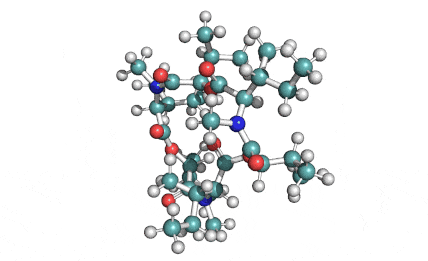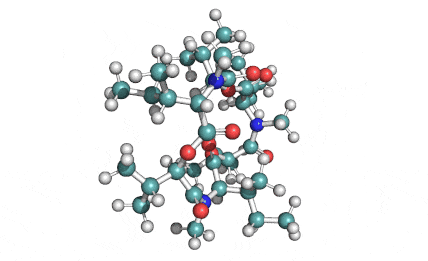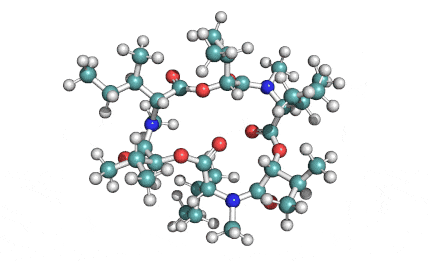
Nonactin
Details
Specifications
Chemical identification
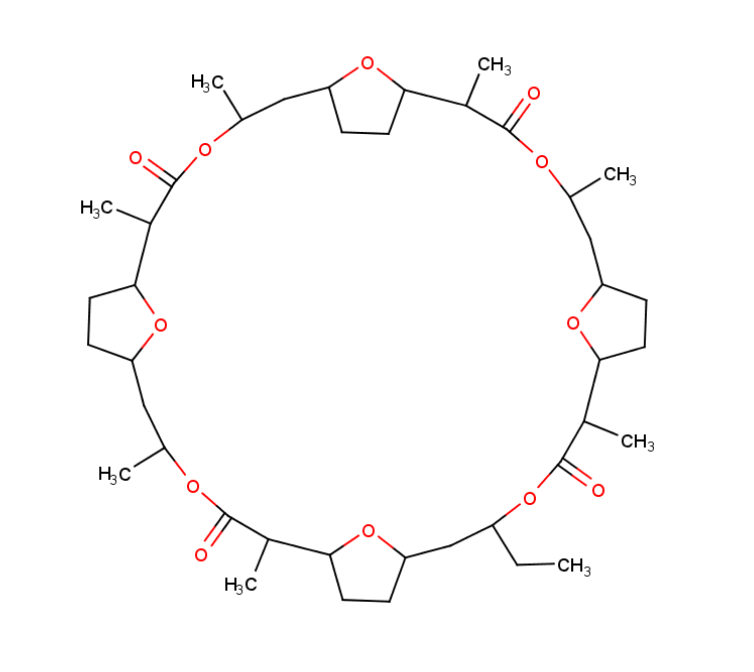
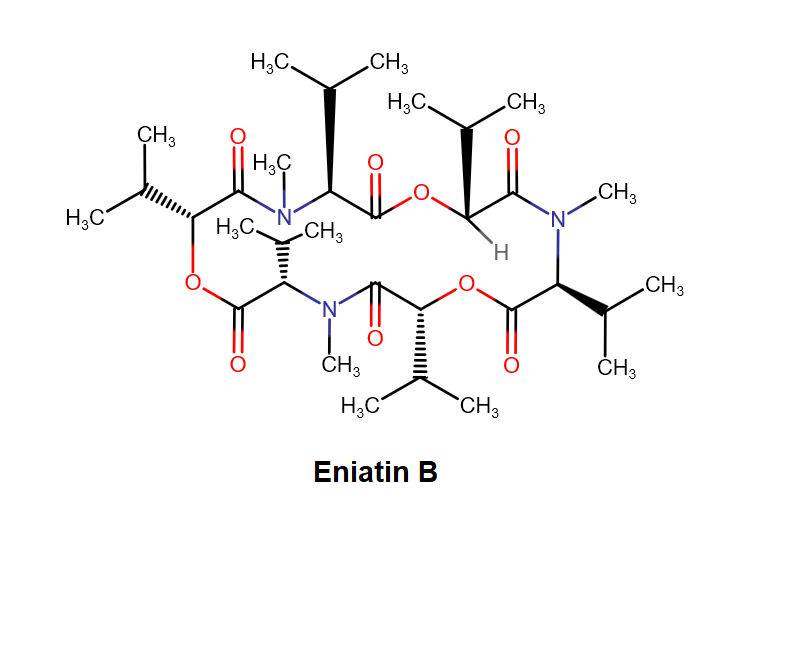
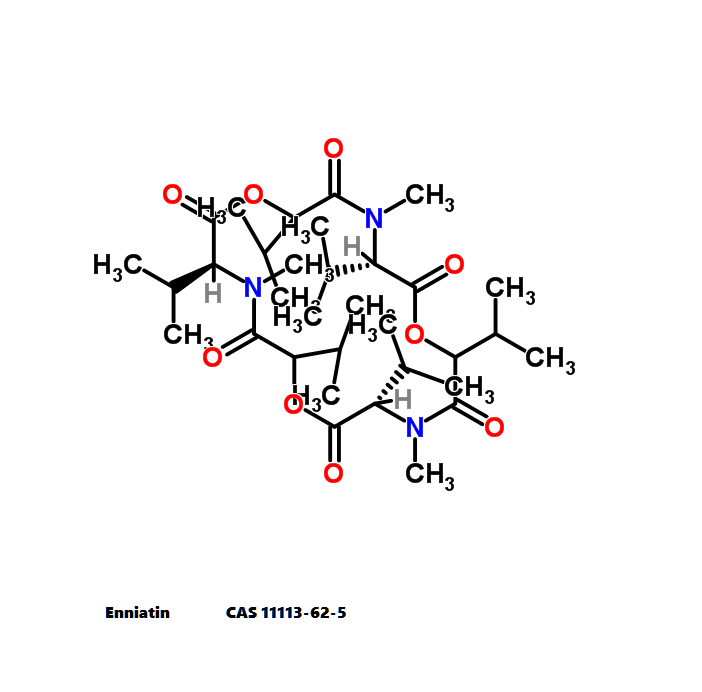
Nonactin is a member of a family of naturally occurring cyclic ionophores known as the macrotetrolide antibiotics. The other members of this homologous family are monactin, dinactin, trinactin, and tetranactin, which are all neutral ionophoric substances and higher homologs of nonactin. Collectively, this class is known as the nactins.
Fermentek started manufacturing of , containing also the lower homologs) in 1993. Since 2025 Fermentek has manufactured pure Nonactin.
The mixture of Nonactin and its homologs is also manufactured and is marketed under the name Nonactin Complex.
Further Information
soluble in Methanol, Dichloromethane, Ethyl Acetate, DMSO. Insoluble in water
Nonactin has an inhibitory effects on the P170 glycoprotein mediated efflux of chemotherapeutic agents in multiple drug resistant cancer cells.
Nonactin was used in agriculture under the name trade Polynactin. Nonactin has been reported to specifically inhibit the processing of cytoplasmic precursor proteins destined for the mitochondria.
Nonactin is used in urea and ammonium specific electrodes.
Composition
Supply related information
Special Info
Other Fields