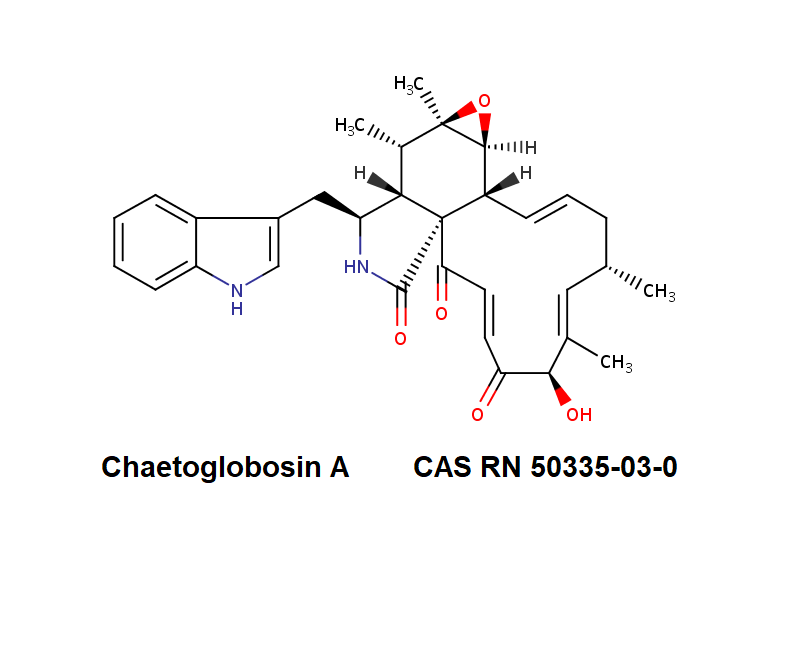
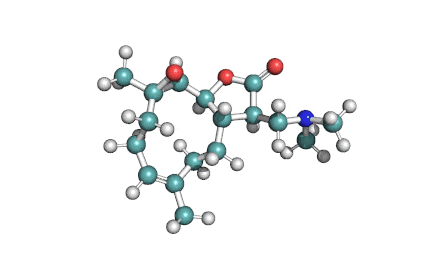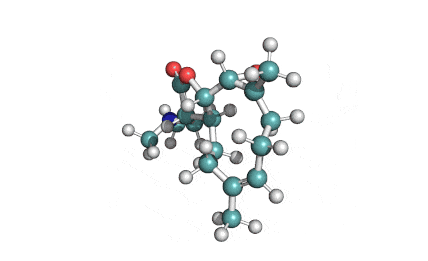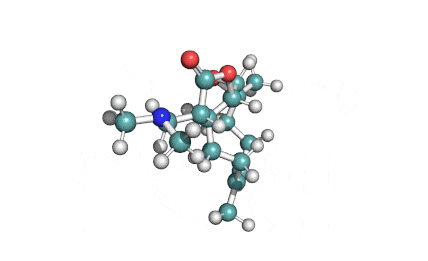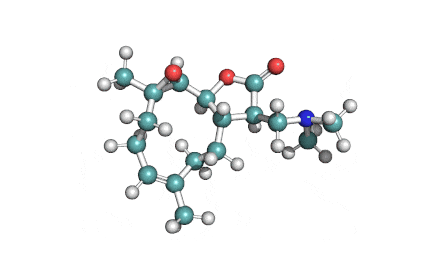
Chaetoglobosin A
Details
Specifications
Chemical identification
Synonyms:
- Chaetoglobosins;
- Chaetoglobosin A;
- CHAETOGLOBOSIN A;
- CHAETOGLOBOSIN;
- CHAETOGLOBOSINB;
Chemical names:
IUPAC:
(1E,4S,5E,7R,9E,11aR,14S,14aR,15S,15aR,16aS,16bR)-7-Hydroxy-14-(1H-indol-3-ylmethyl)-4,6,15,15a-tetramethyl-4,7,14,14a,15,15a,16a,16b-octahydro-3H-cyclotrideca[d]oxireno[f]isoindole-8,11,12(13H)-trion e
RTECS#:
- HA530500
Antibiotic agent.
Phytotoxin. Cytochalasin analog
- Targets filamentous actin and induces apoptosis.
- Shows cytotoxic effects against cancer cell lines.
- Shows antibacterial and nematicidal effects.
- Induces tissue necrosis in vivo
Further Information
Soluble in DMSO , Ethanol, Methanol
Chemical classification:
- Indole Alkaloids
Bioactivity classiification:
- antibacterial
- cytotoxic
- Apoptosos inducer
Composition
Special Info
Other Fields