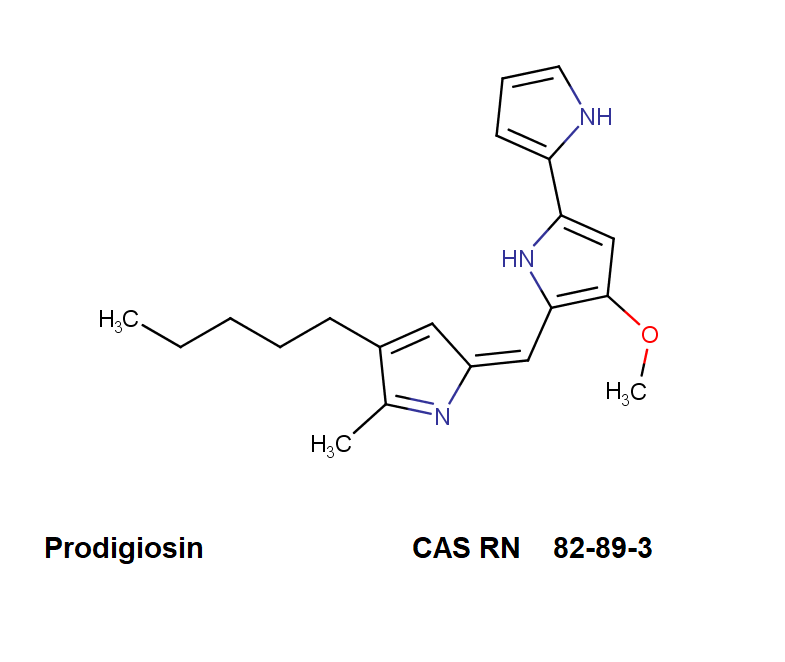
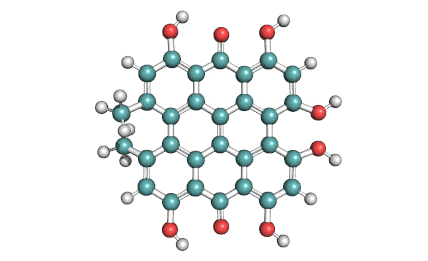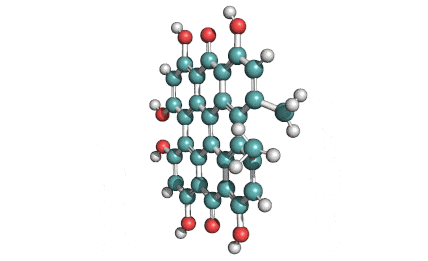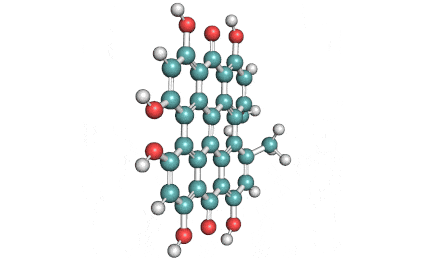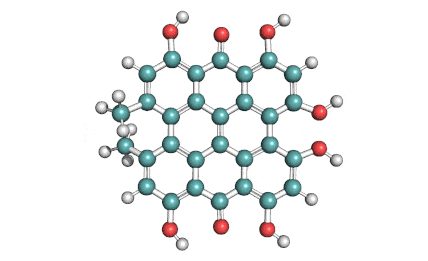
Prodigiosin hydrochloride
Details
Specifications
Chemical identification
Synonyms:
Disambiguation: Do not confuse with Prodigiozan, another antibiotic from the same source.
Chemical names: 2,2'-Bi-1H-pyrrole, 4-methoxy-5-[(5-methyl-4-pentyl-2H-pyrrol-2-ylidene)methyl]-, hydrochloride (1:1)
IUPAC: (2Z)-3-methoxy-2-[(5-methyl-4-pentyl-1H-pyrrol-2-yl)methylidene]-5-(1H-pyrrol-2-yl)pyrrole;hydrochloride
RTECS DW2977000 refers to prodigiosin free base
Prodigiosin is a antibiotic from Serratia marcescens and some other bacterial species. It displays a wide range of biological activities, making it a promissing candidate drug. Among these are antimalarial, antifungal, anti-cancer, immunosuppressant, and antibiotic properties.
Further Information
(Data from literature including scientific publications and books, patents and other vendors)
Prodigiosin is soluble in acetonitrile, ethanol, methanol, chloroform, and DMSO. It is insoluble in water.
UNSPSC
- 51000000 Drugs and Pharmaceutical Products
- 51200000 Immunomodulating drugs
For research only!
Composition
Supply related information
Special Info
Other Fields