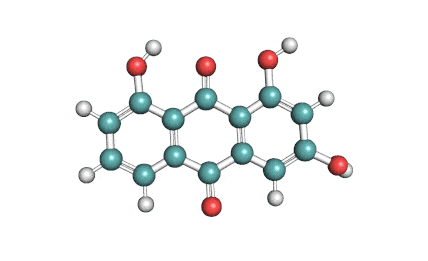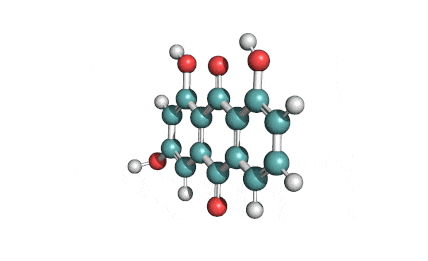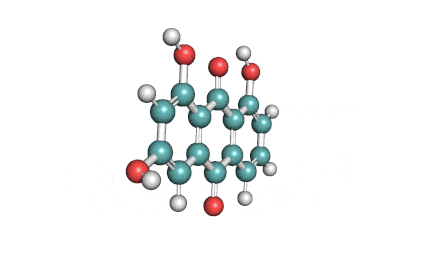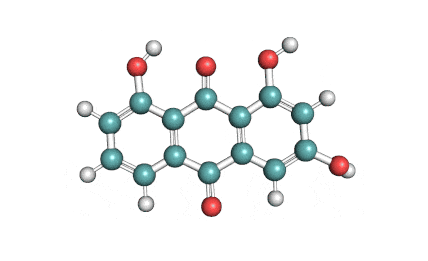
Emodin
Details
Specifications
Clear yellow to red solution at 10mg/ml of Ethanol (may need a little warming)
Chemical identification
Synonyms
- Archin
- Emodol
- Frangula emodin
- Frangulic acid
- Rheum emodin
Chemical names Emodin
RTECS CB7920600
Emodin is an anthraquinone class compound. It is sourced from a plant Rheum palmatum. It exhibits antibacterial, anti-neoplastic, anti-inflammatory and anti-angiogenesis properties.
Further Information
Water Solubility <0.1 g/100 mL at 19ºC ; Practically insoluble in water; soluble in alcohol, aqueous alkali hydroxide solutions (cherry red color), (src: Merck index)
Emodin has been investigated for the treatment of Polycystic Kidney.
Composition
Supply related information
Special Info
Other Fields