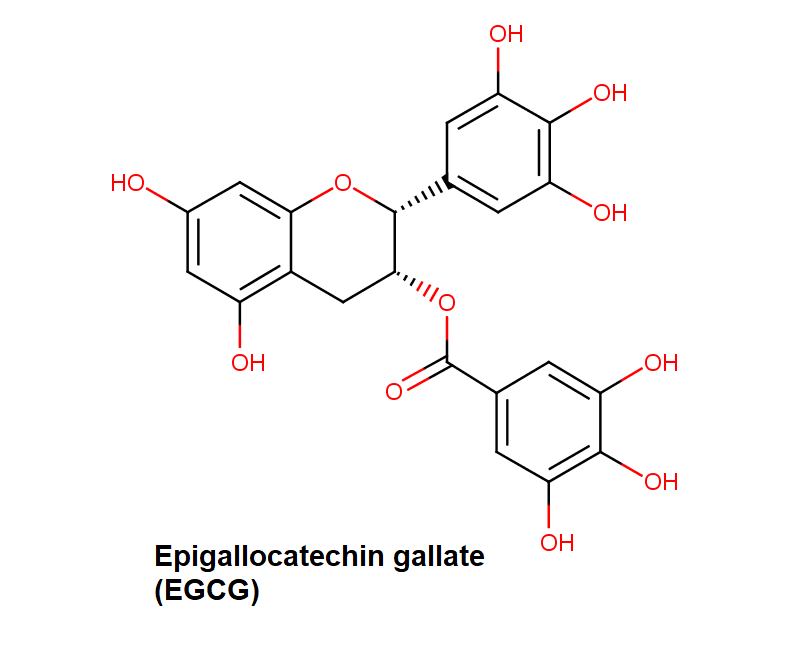
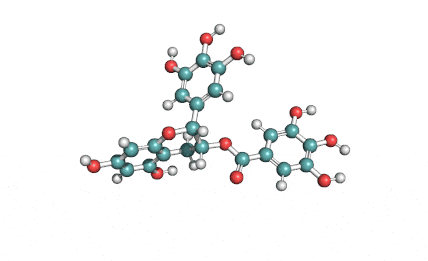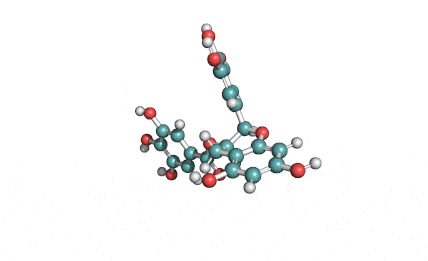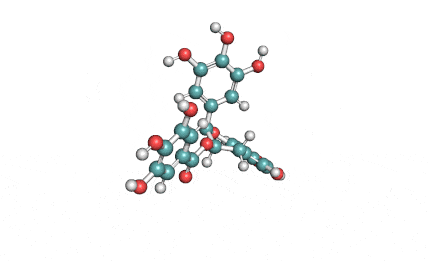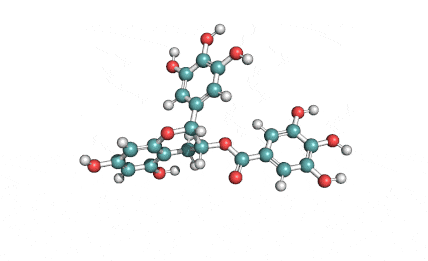
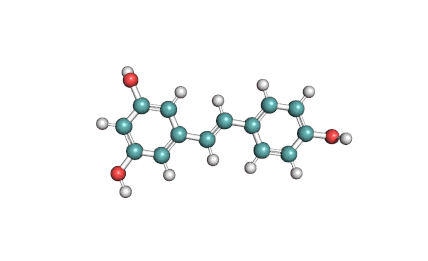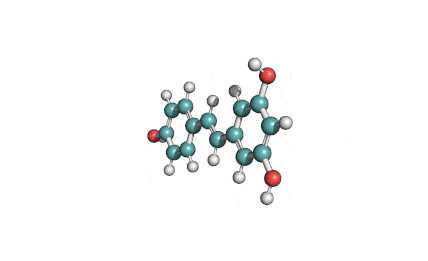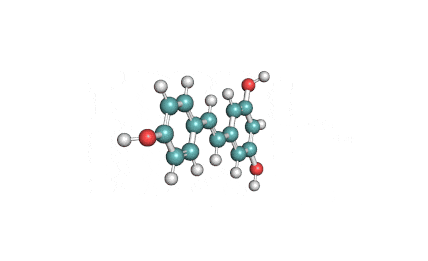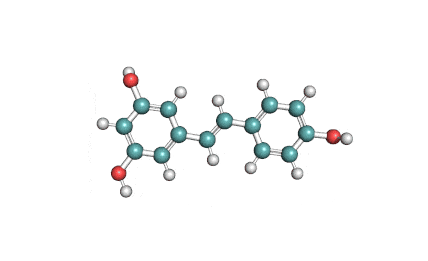
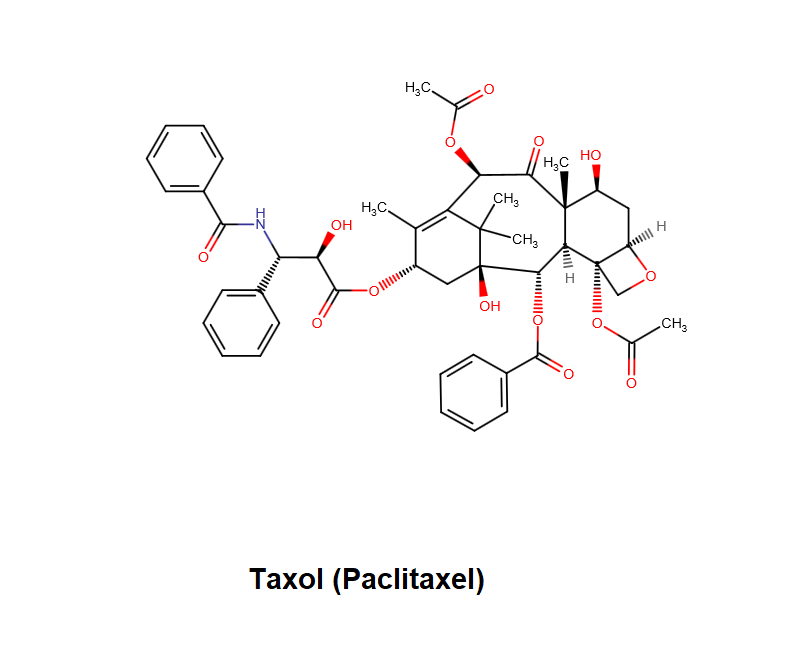
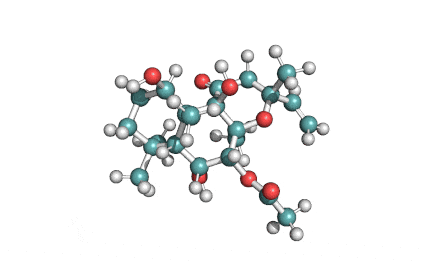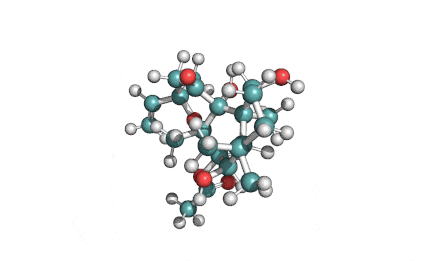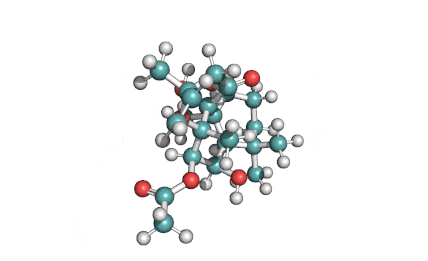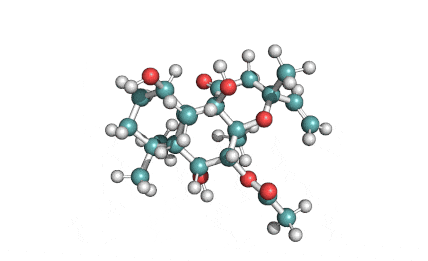
Epigallocatechin Gallate
Details
Specifications
Chemical identification
RTECS KB5200000
Epigallocatechin is a phenolic antioxidant found in a number of plants such as green and black tea.
Epigallocatechin inhibits cellular oxidation and prevents free radical damage to cells.
Further Information
- Antioxidant
- Potential cancer-preventive agent.
- Inhibitor of Telomerase
Epigallocatechin is being studied as a potential cancer chemo preventive agent.
Composition
Special Info
Other Fields