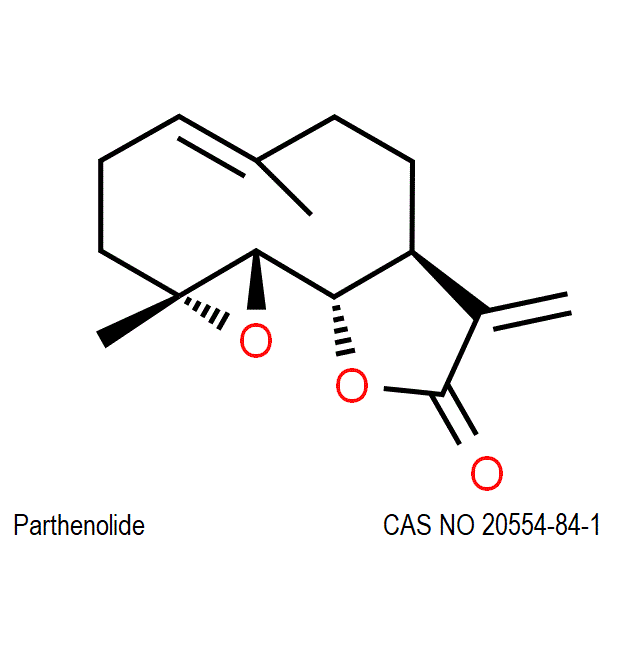
Parthenolide
Molecular Formula
C15H20O3
M.W.
248.32
CAS number
20554-84-1
MSDS
Details
Source
Tanacetum parthenium
Fermentek product Code
PAR-001
Brand/grade
For research
Specifications
Appearance
White to off white powder
Purity by HPLC
≥98% ; refer to CoA for more data
Purity By TLC
≥98% ; refer to CoA for more data
Solubility test
Clear colorless to faint yellow solution at 50mg/ml Dichloromethane; Clear colorless to faint yellow solution at 50mg/ml DMSO
Chemical identification
Names and identifiers
IUPAC name: (1aR,4E,7aS,10aS,10bS)-1a,5-Dimethyl-8-methylene-2,3,6,7,7a,8,10a,10b-octahydrooxireno[9,10]cyclodeca[1,2-b]furan-9(1aH)-one
RTECS: LY4220000
EU number
692-532-0
Chemical name
Parthenolide
Description
Parthenolide is a sesquiterpene lactone and active principle of feverfew (Chrysanthemum parthenium)
InChl Key
KTEXNACQROZXEV-PVLRGYAZSA-N
Canonical SMILES
CC1=CCCC2(C(O2)C3C(CC1)C(=C)C(=O)O3)C
Isomeric SMILES
C/C/1=C\CC[C@@]2([C@H](O2)[C@@H]3[C@@H](CC1)C(=C)C(=O)O3)C
Further Information
Solubility ( literature )
DMSO (100 mg/ml), Ethanol (20 mg/ml), Dichloromethane.
Compound Classification
- sesquiterpene lactone
MAP kinase inhibitor
Storage, handling
Store in a freezer upon arrival, at -10°C to -25°C
Applications
Parthenolide has anti-inflammatory, antisecretory and spasmolytic activity. It Inhibits the release of various mediators. It inhibits activation of MAP kinase.
Disclaimer
For Research use only
Not for Human or Drug use
Not extracted from humans or animals
Refer to MSDS for further safety and handling instructions
Composition
Ingredient type
Fermentek product
Special Info
Available since
Other Fields
Title
Transport information
Value
Not hazardous for transport
Fermentek Product Category
Signal to sort
P
Image
Image
Image

Image