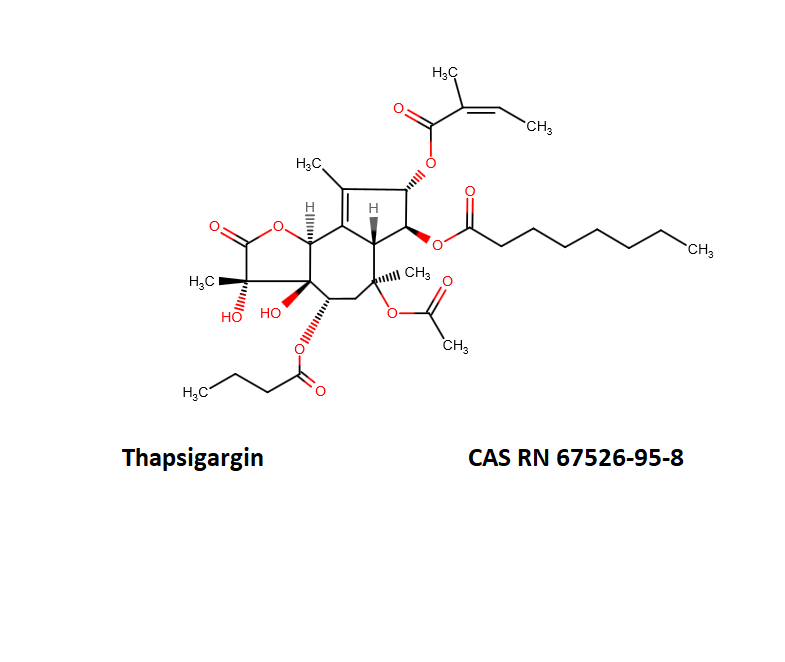
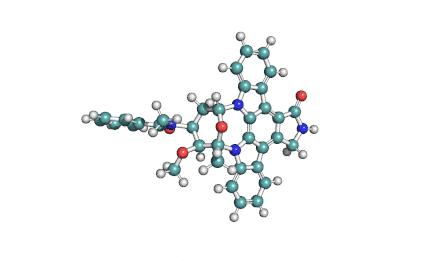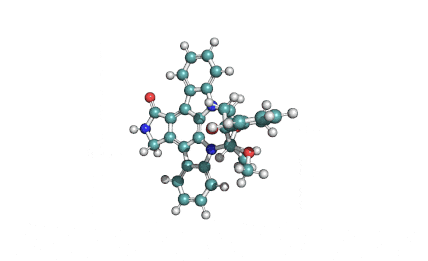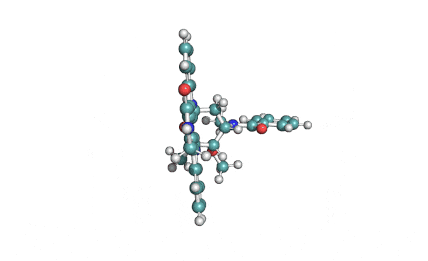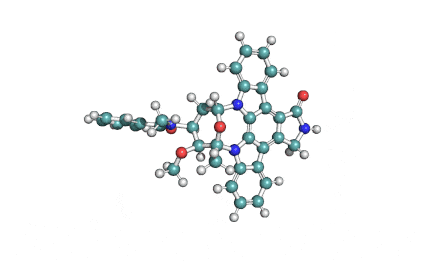
Thapsigargin
Details
Specifications
Chemical identification
Synonyms: octanoic acid {3S-[3alpha,3abeta,4alpha,6beta,6abeta,7beta,8alpha(Z),9balpha]}-6-(acetoxy)-2,3,3a,4,5,6,6a,7,8,9b-decahydro-3,3a-dihydroxy-3,6,9-trimethyl-8-[(2-methyl-1-oxo-2-butenyl)oxy]-2-oxo-4-(1-oxobutoxy)-azuleno[4,5-b]furan-7-yl ester
RTECS RH0352700
Thapsigargin is a cell-permeable, tumor promoting sesquiterpene lactone. A tight-binding inhibitor of intracellular calcium (SERCA) pumps (sarco/endoplasmic reticulum Ca2+ ATPase). Chemically it is classified as a sesquiterpene lactone antibiotic.
A study from the University of Nottingham showed promising results for its use against Covid-19 and other coronavirus.
Further Information
DMSO, Acetonitril, Ethanol, Methanol, Dichloromethane
- Sesquiterpene lactone
- SERCA inhibitor
- Apoptosis related antibiotic
Composition
Special Info
Other Fields