beta Zearalanol
Details
Specifications
Chemical identification
Synonyms:
- Estrogen P 1560
- Taleranol
Chemical names:
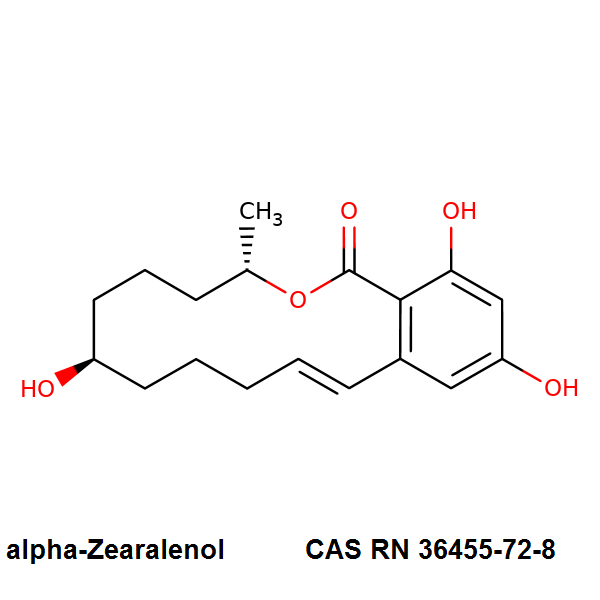
- β- Zearalanol
IUPAC:
- (7S,11S)-7,15,17-trihydroxy-11-methyl-12-oxabicyclo[12.4.0]octadeca-1(14),15,17-trien-13-one
RTECS# DM2540000
Beta Zearalanol, also known as taleranol, is a minor analogue of the zearalenone family of resorcinyl macrocyclic lactones, produced by several species of Fusarium. Like the other zearalenones, Beta-zearalanol exhibits estrogenic activity in animals.
Further Information
Composition
Other Fields