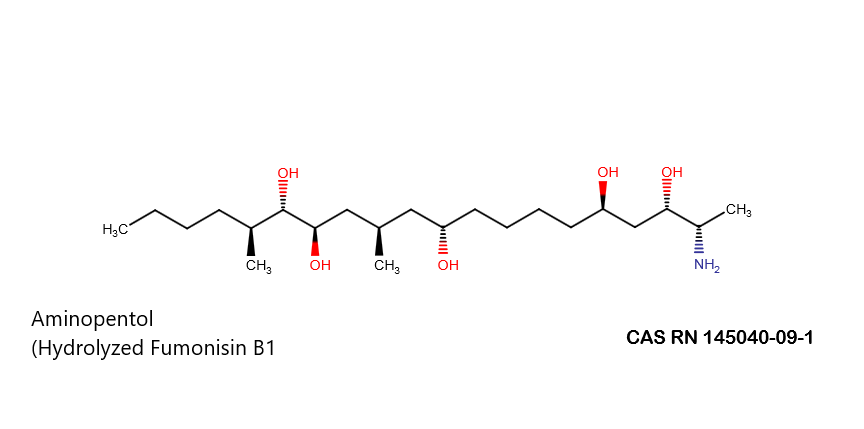
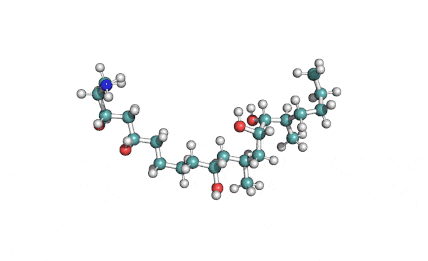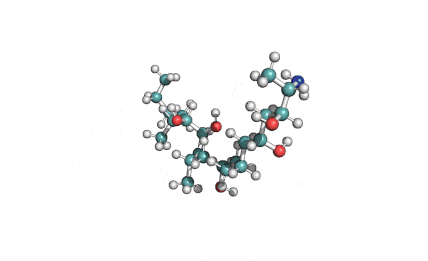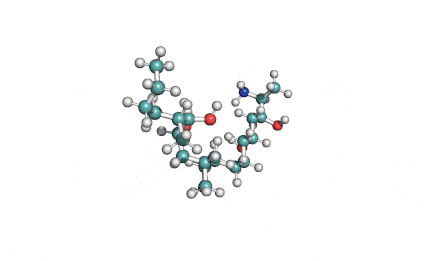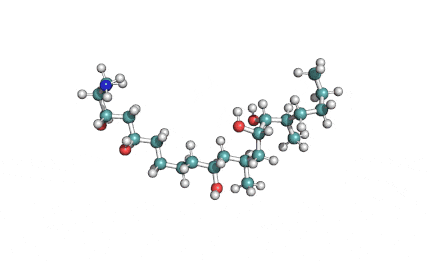
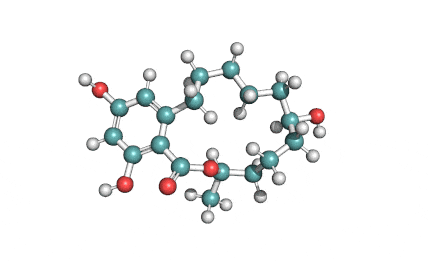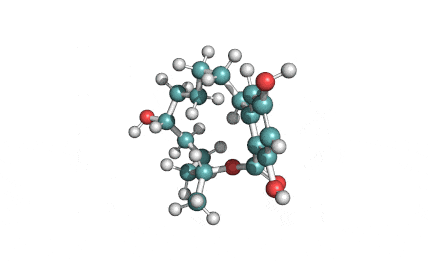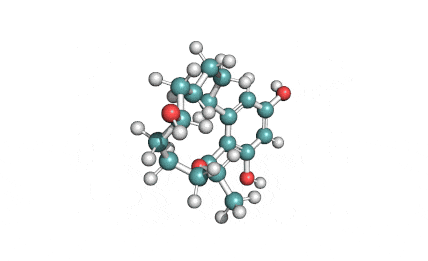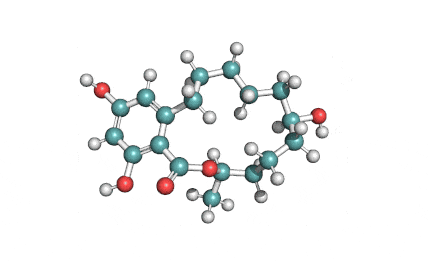
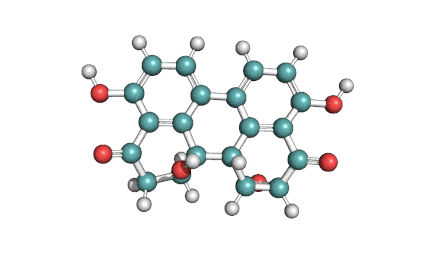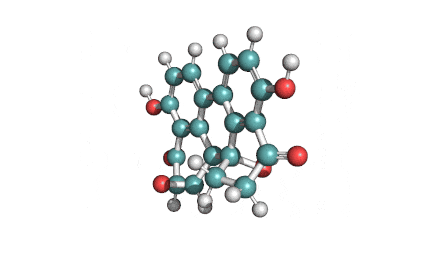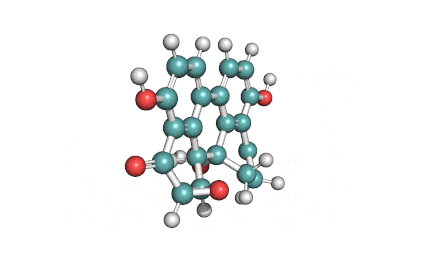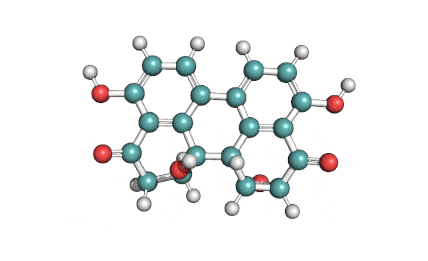
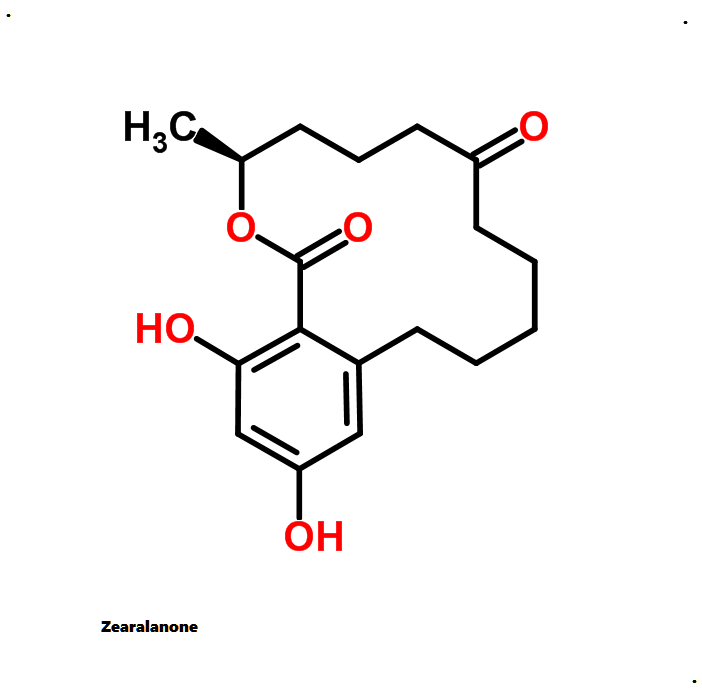
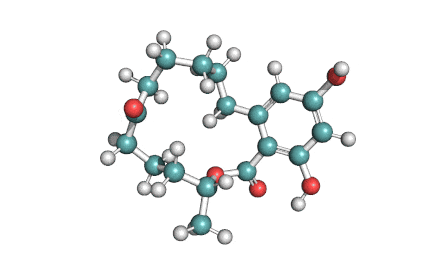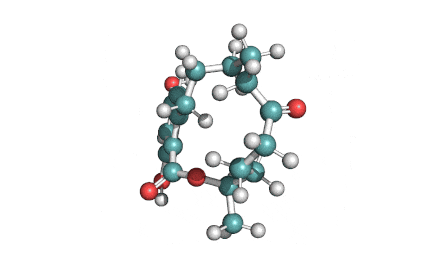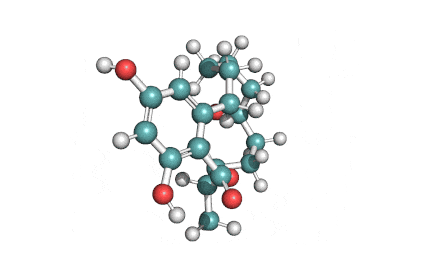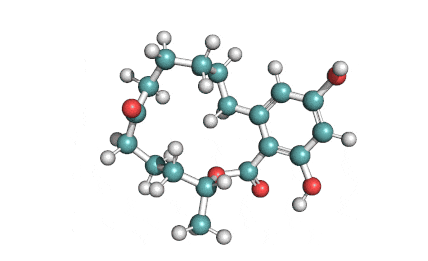
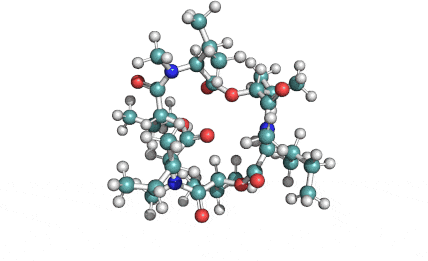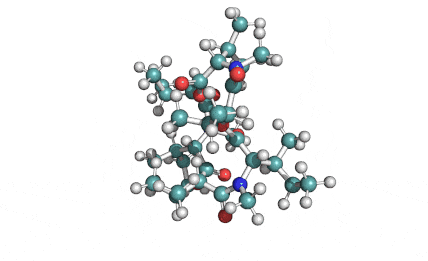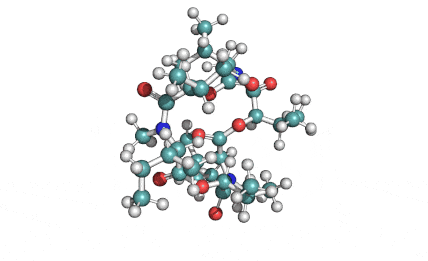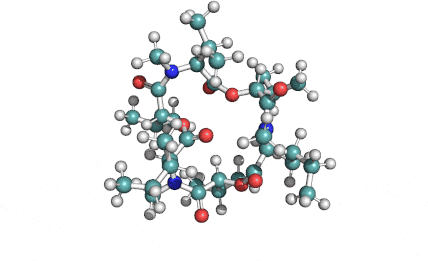
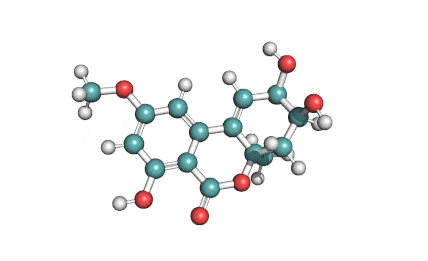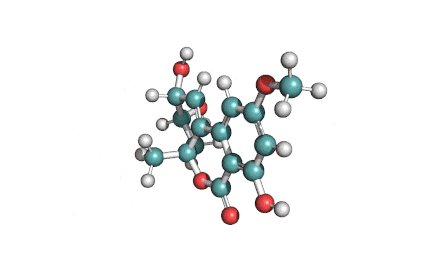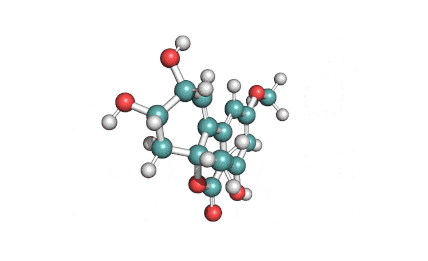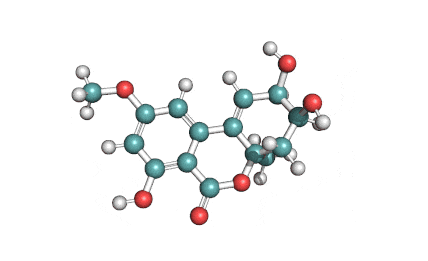
Hydrolyzed Fumonisin B1 (Aminopentol)
Details
Specifications
Chemical identification
Synonyms:
- Aminopentol;
- AP1
Chemical names:
IUPAC:
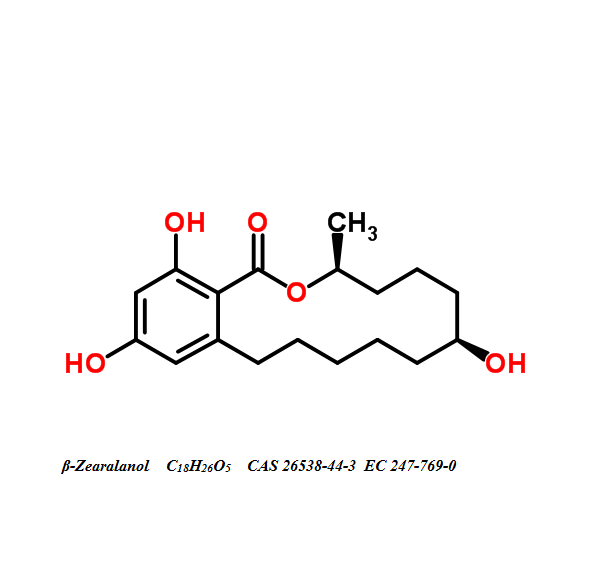
- (2S,3S,5R,10R,12S,14S,15R,16R)-2-Amino-12,16-dimethyl-3,5,10,14,15-icosanepentol
RTECS#
- not available.
Hydrolyzed fumonisin B1 (aka aminopentol) is a mycotoxin which occurs naturaly together with fumonisin B1. It may be formed from fumonisin B1 by hydrolysis of the O-acyl bonds.
Further Information
NotGMO
Product natural
Composition
Supply related information
Special Info
Other Fields