Diacetoxyscirpenol (DAS)
Details
Specifications
Chemical identification
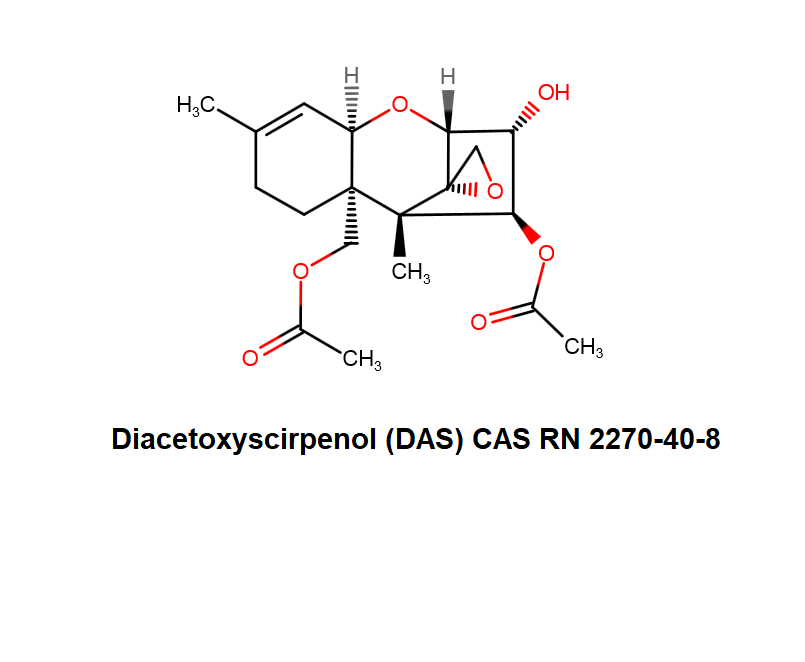
Diacetoxyscirpenol (DAS) also known as Anguidine, is a mycotoxin belonging to 12,13-epoxytrichothecene group, produced by several Fusarium strains together with some other toxins such as T2 and HT2 toxins.
DAS inhibits initiation of protein synthesis, causing the death of intensively replicating cells. By the virtue of this effect, DAS possesses teratogenic and anti proliferatory properties. Consequently, DAS attracts interest as a potential cancer- drug.
Further Information
Soluble in moderately polar solvents, such as chloroform, diethyl ether, ethyl acetate, and acetone
- Trichothecene mycotoxin
- Apoptosis inducer
Diacetoxyscirpenol (Anguidine) and its derivates possess anticancer properties. Diacetoxyscirpenol (Anguidine) inhibits initiation of protein synthesis, resulting in the death of rapidly proliferating cells. Diacetoxyscirpenol (Anguidine) also has been shown to both potentiate and protect against the cytotoxic effects of other drugs. There were 14 clinical trials reported, all in the area of colorectal tumors and leukemia. No records found after 1985. Apoptosis inducement.
Composition
Special Info
Other Fields