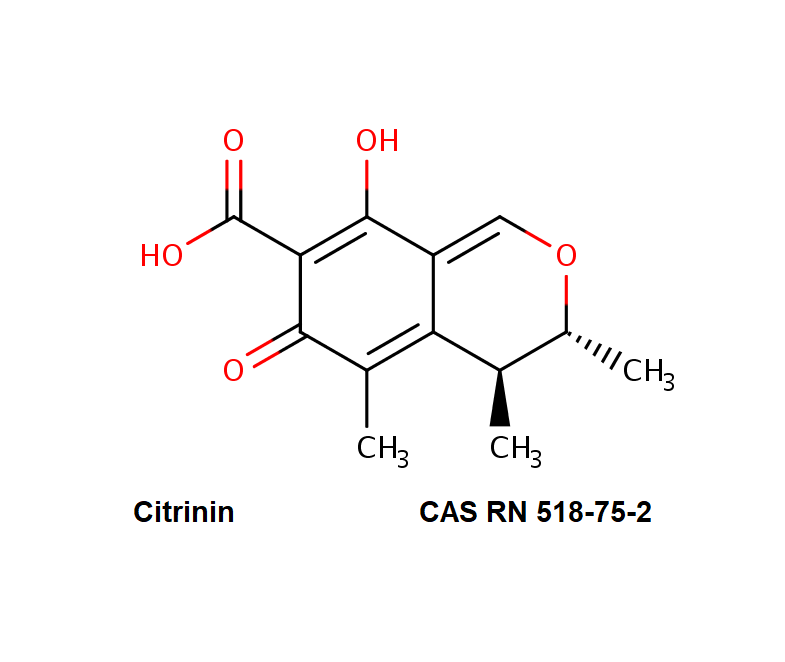
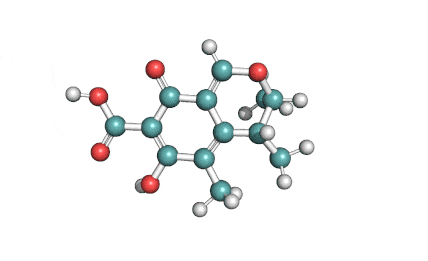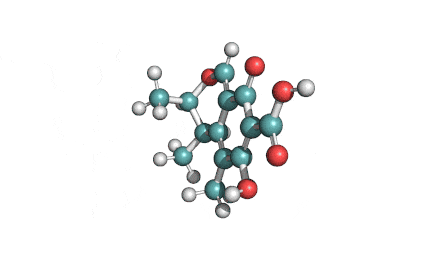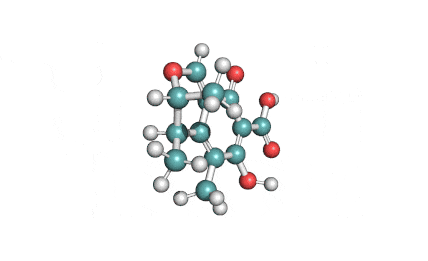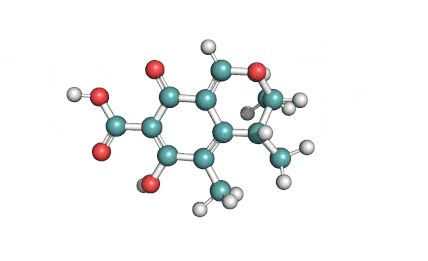
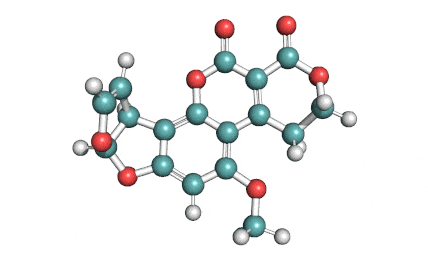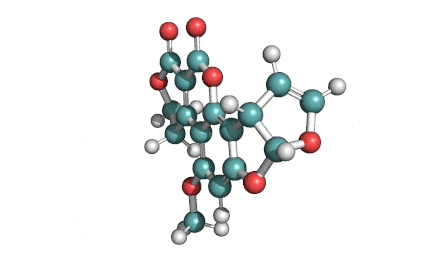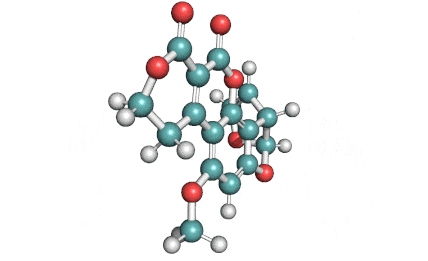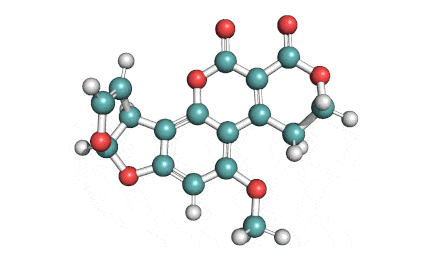
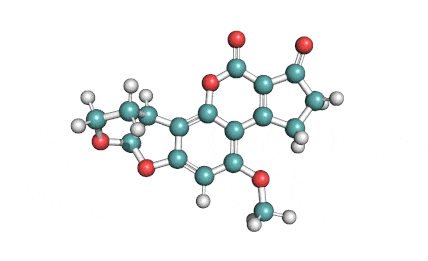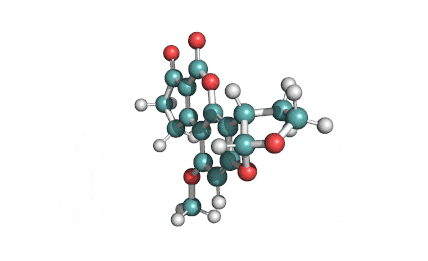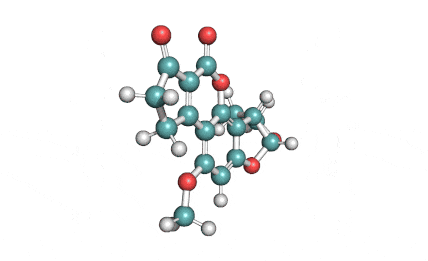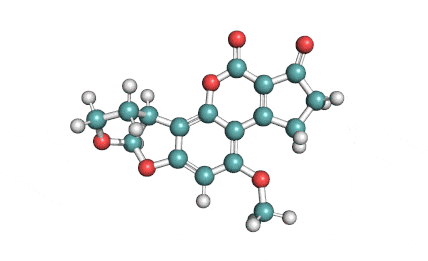
Citrinin
Details
Specifications
Chemical identification
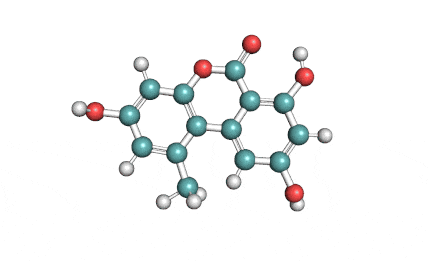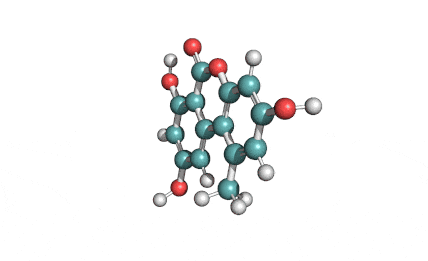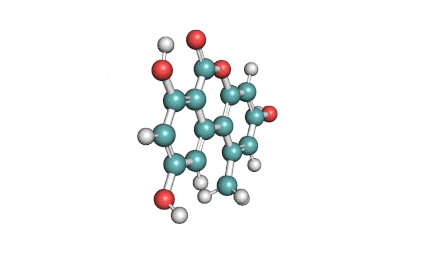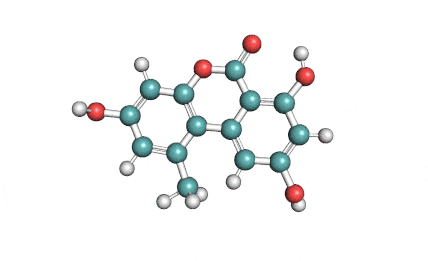
IUPAC Name (3R,4S)-8-Hydroxy-3,4,5-trimethyl-6-oxo-4,6-dihydro-3H-isochromene-7-carboxylic acid
RTECS DJ2275000
Citrinin is a mycotoxin capable of inducing mitochondrial permeability transition. Citrinin also inhibits microtubule polymerization
Further Information
Slightly soluble in water. Soluble in dilute alkaline solutions. Soluble in methanol, ethanol, acetonitrile
Benzopyran mycotoxin
Apoptosis inducer
Initially isolated as a broad-spectrum antibiotic.
Composition
Special Info
Other Fields