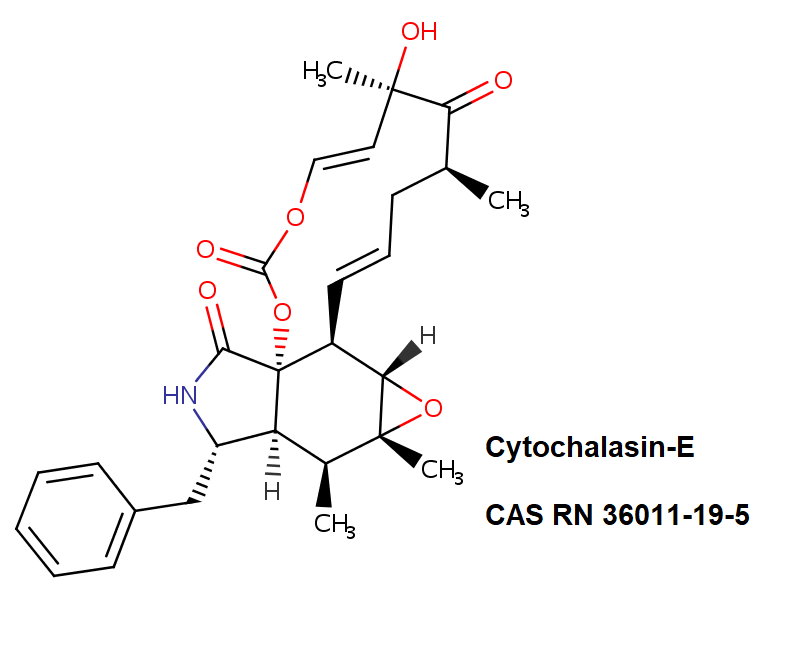
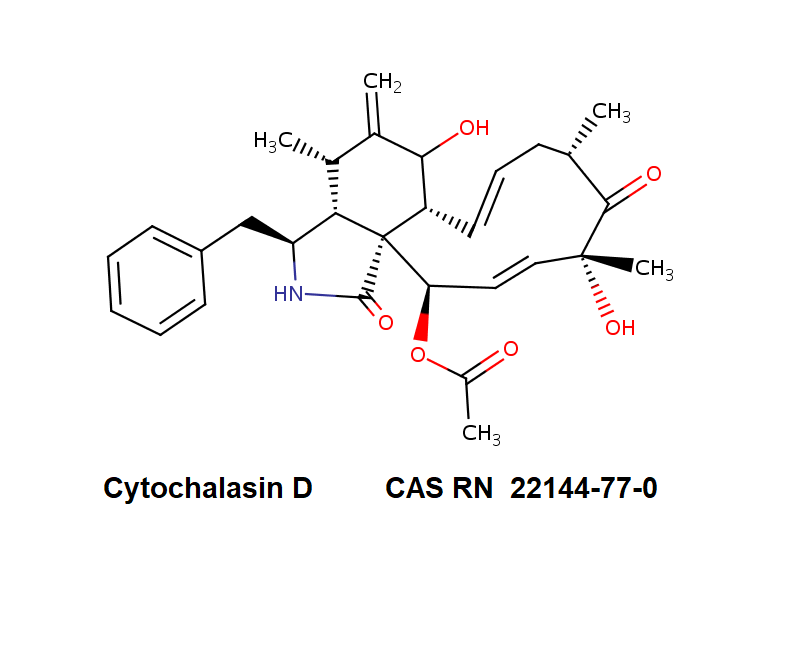
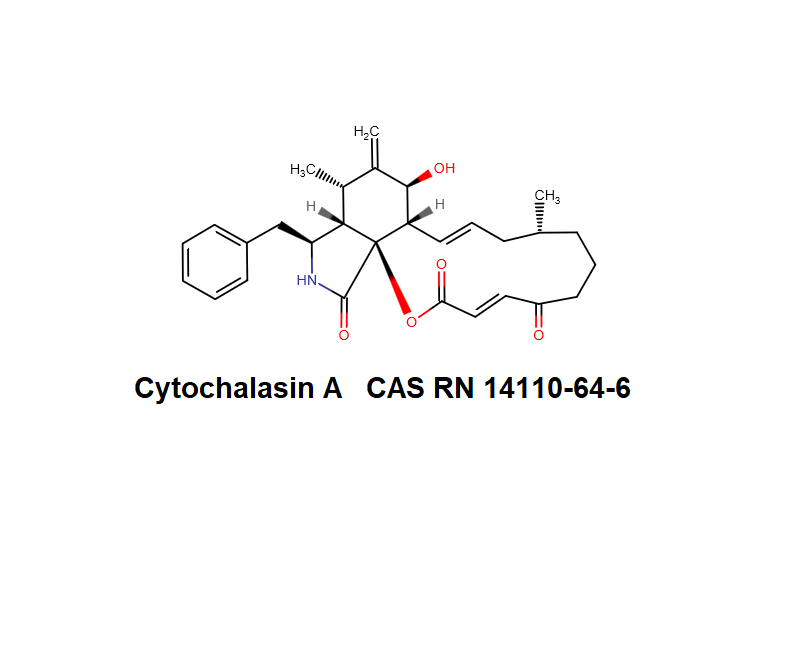
Dihydrocytochalasin B
Details
Specifications
Chemical identification
Synonyms:
- 21,22-Dihydrochalasin B
- 21,22-Dihydrocytochalasin B
- 21,22-Dihydrophomin
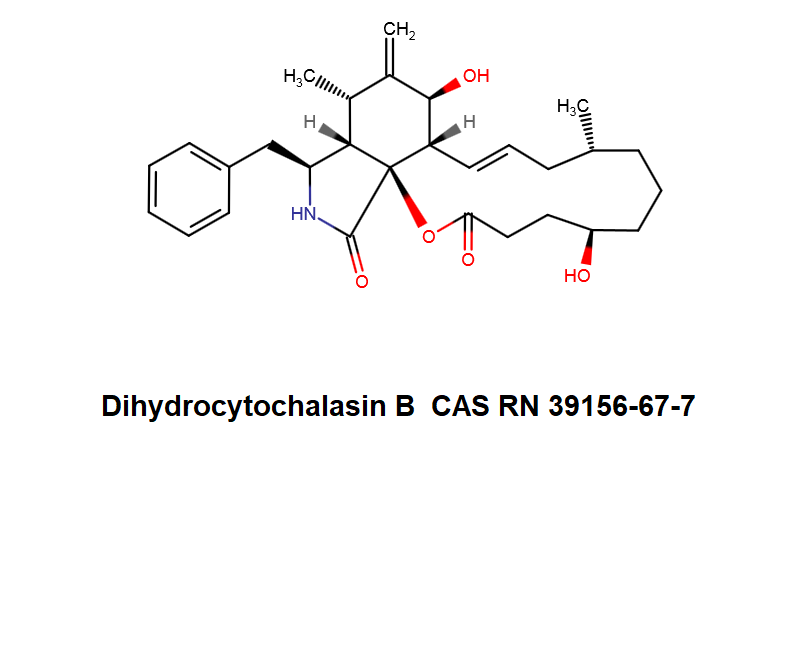
Systematic Name: 7(S),20(R)-Dihydroxy-16(R)-methyl-10-phenyl-24-oxa(14)cytochalasa-6(12),13(E)-diene-1,23-dione
Dihydrocytochalasin B is chemically derived from Cytochalasin B obtained natural from Drechslera dematoidea fungus.
Further Information
Acetone, DMSO, 100% Ethanol, Methanol
- Macrolide indol mycotoxin
- Cytochalasin
- Actin inhibitor
Cytochalasins are used as tools in cytological research, and in in the field of actin polymerisation.
Cytokinesis inhibitor. Inducer of changes in cell morphology and motility. It disrupts actin structure and inhibits ability of serum growth factors to stimulate DNA synthesis in vitro. Does not inhibit sugar uptake. Active calcium transport inhibitor. Active in vivo.
Composition
Special Info
Other Fields