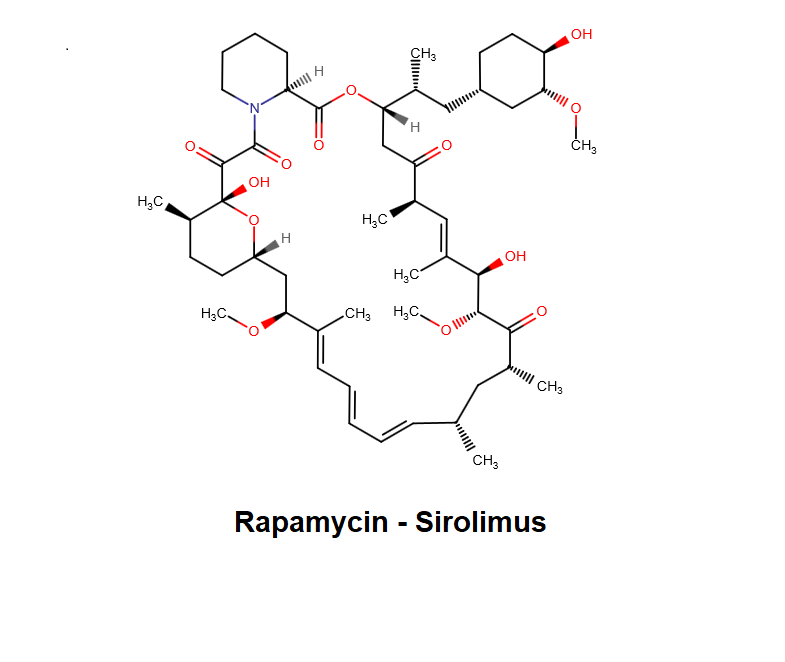
Rapamycin
Details
Specifications
Chemical identification
Synonyms: RAPA, Rapamune, Sirolimus, RPM, Antibiotic AY 22989
RTECS: VE6250000
Rapamycin (Sirolimus) is a macrolide compound that acts by selectively blocking the transcriptional activation of cytokines thereby inhibiting cytokine production. It is bioactive only when bound to IMMUNOPHILINS. Sirolimus (Rapamycin) is a potent immunosuppressant. Sirolimus (Rapamycin) possesses both antifungal and antineoplastic properties.
Further Information
Methanol, DMSO
macrolide immunosuppressor
Research applications: Rapamycin Sirolimus inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Rapamycin Sirolimus inhibits T-lymphocyte activation / proliferation occuring in response to antigenic and cytokine (Interleukin IL-2, IL-4, and IL-15) stimulation by a mechanism that is distinct from that of other immunosuppressing agents. Sirolimus also inhibits antibody production.
Composition
Special Info
Other Fields