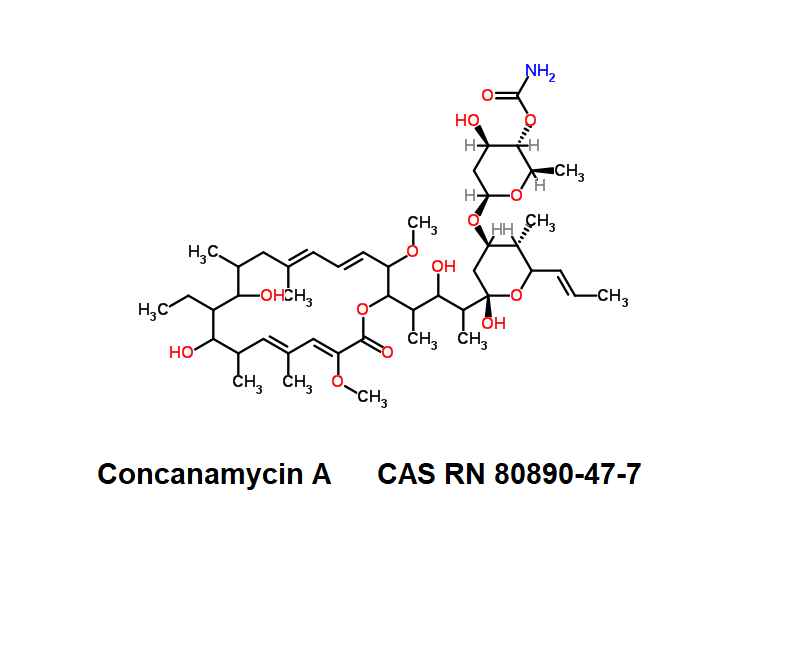
Ansamitocin P3
Details
Specifications
Chemical identification
Names and synonyms
- Maytansinol ISO-butyrate
- Antibiotic C15003P3
- Ansamitocin P-3
- Ansamitocin P3
RTECS: OQ2291000
Fungal metabolite with antimitotic, antineoplastic activity. Ansamitocin P3 binds to tubulin and inhibits vinblastine-induced spiral formation
Further Information
Composition
Special Info
Other Fields